Abivax 2018 Financial Results and Operations Update
ABX464 showed impressive safety and efficacy during induction treatment of Ulcerative Colitis (UC)
Magnitude and durability of efficacy further increased after 6 months treatment during open-label maintenance study
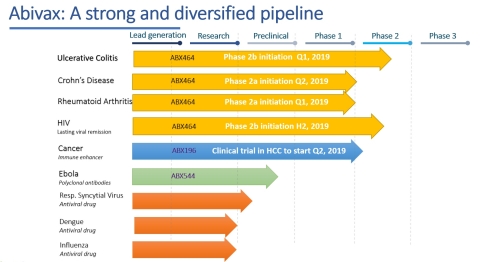
ABX464 advancing into Phase 2b trials in UC and Phase 2a for Crohn’s disease and Rheumatoid Arthritis
ABX196 to file US IND for first clinical trial in patients with hepato-cellular carcinoma
Two RSV lead compounds from mRNA Discovery Platform advancing into pre-clinical development
Available funding, up to
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20190313005922/en/
“2018 was a terrific year for
“In addition, ABX464’s unique mechanism of action, preclinical and clinical data suggest a broadly applicable anti-inflammatory effect, which has prompted the preparation of Phase 2a clinical trials of ABX464 in Crohn’s disease and rheumatoid arthritis, to be initiated in the coming months. We are confident we can build strong shareholder value with these achievements,” Professor Ehrlich continued.“Furthermore, an IND for our clinical trial with ABX196 in hepatocellular carcinoma patients will be submitted shortly to the US FDA. And finally, two lead compounds have been identified for the prophylaxis and/or treatment for patients with respiratory syncytial virus infection. Given this exciting R&D portfolio, the company is clearly prioritizing ABX464 in inflammatory indications, as well as ABX196, while other programs (e.g. Ebola) will be put on hold”.
2018 FINANCIAL HIGHLIGHTS
| Items in the Income Statement in millions of euros |
FY 2018 | FY 2017 | Variance | |||
| Total operating income | 0.8 | 0.4 | 0.4 | |||
| Total operating expenses | (19.9) | (14.5) | (5.4) | |||
| of which Research and Development costs | (15.9) | (10.8) | (5.1) | |||
| of which administrative costs and overheads | (4.0) | (3.7) | (0.3) | |||
| Operating result | (19.1) | (14.1) | (5.0) | |||
| Financial result | (0.5) | 0.0 | (0.5) | |||
| Ordinary result | (19.6) | (14.1) | (5.5) | |||
| Extraordinary result | 0.0 | 0.2 | (0.2) | |||
| Tax on income | 3.8 | 2.7 | 1.1 | |||
| Result for the period | (15.8) | (11.2) | (4.6) | |||
- Operating loss €19.1m (- €5.0m compared to - €14.1m as of
December 31, 2017 ) mainly reflects the increasing investments in R&D (+ €5,1m) - Total number of employees at the end of
December 2018 was steady at 25 - R&D expenses amounted to €15.9m, mainly due to the development of ABX464 in inflammatory indications (69%), as well as investments in the progressive scaling up of the mRNA splicing platform and library of small molecules (23%)
- G&A expenses were at €4.0m in 2018 (20% of total operating costs) compared to €3.7m (26%) in 2017
- Revenues, which were comprised mainly of a Research Tax Credit, were at €3.8m in 2018, compared to €2.7m in 2017
- The Company’s cash utilization rate during 2018 was €1.5m per month
- Cash at the end of 2018 was €13.0m, compared to €17.0m at the end of 2017
- Company is fully funded through Q1 2020, based on the following assumptions:
- the assessment of planned R&D needs
- the €10m second tranche of
Kreos Capital , which has been amended inJanuary 2019 , with a drawing bound to the start of Ulcerative Colitis Phase 2b clinical trial before midJuly 2019 (Tranche B). The €10m first tranche ofKreos Capital loan agreement was drawn inJuly 2018 (Tranche A) - the exercise of the remaining equity line with Kepler Cheuvreux for €7m (€9 share price assumption)
- the 2019 cash in resulting from 2018 Research Tax Credit and 2018 Bpifrance RNPVir milestone, which together are planned at €5m
| Financial Items from the Balance Sheet
in millions of euros |
12/31/2018 | 12/31/2017 | Variance | |||
| Net financial position | 2.1 | 16.8 | (14.7) | |||
| of which financial fixed assets* | 5.0 | 15.0 | (10.0) | |||
| of which fixed-term deposits (maturing in > 1 year) | 0.0 | 0.0 | 0.0 | |||
| of which fixed-term deposit (maturing in <1 year) | 5.0 | 15.0 | (10.0) | |||
| of which available cash flow | 8.0 | 2.0 | 6.0 | |||
| (of which financial debts) | (10.9) | (0.3) | (10.6) | |||
| Total assets | 54.0 | 53.8 | 0.2 | |||
| Total equity | 34.7 | 48.2 | (13.6) | |||
| of which equity capital | 28.7 | 43.9 | (15.2) | |||
| of which conditional advances | 5.9 | 4.3 | 1.6 | |||
|
* Excluding items of the liquidity contract (liquidity and own shares) and deposits & guarantees |
||||||
Operating Highlights: Portfolio Update
ABX464 in UC and other inflammatory diseases
In September of 2018,
At the end of the completed 2-month induction study in 32 patients, 22 of these (15 previously treated with ABX464 and 7 who had received placebo) opted to enroll in the 12-month open-label maintenance study, ABX464-102. At month six, 19 of the 22 patients were still in the study, receiving a once-daily, oral capsule of 50mg ABX464. The 6-month interim analysis showed that ABX464 continued to have a good safety profile when administered chronically. The efficacy data as assessed by partial
_______________
1 The partial
2 The total
Importantly, the reduction in pMS was correlated with a major reduction of fecal calprotectin, the most widely used biomarker in UC. During the maintenance study at month 6, fecal calprotectin was reduced by an overall of 98% versus baseline in patients on ABX464 during induction (68% after 2 months induction and 30% during maintenance), and by 91% in former placebo patients. Importantly, the mean fecal calprotectin levels in the 2 groups decreased to 86 and 54 ug/g respectively, and thus very close to normal values, which are in the range of up to 50 ug/g for individuals with no IBD, between 50 and 200 ug/g for borderline cases, and above 200 ug/g for patients with Inflammatory Bowel Disease (IBD).
The inflammatory disease space represents an area of high unmet medical need, and a corresponding substantial market opportunity. It is estimated that nearly 1 million patients with ulcerative colitis live in the US, 650,000 in
ABX464 clinical development in HIV
In June of 2018,
ABX196 – a clinical stage immune enhancer for oncology based on iNKT regulation
ABX196 is a synthetic agonist (glycolipid) of iNKT (invariant Natural Killer T) cells, in a liposomal formulation. Preclinical development of ABX196 has shown its capacity to turn tumors that are non-responsive to checkpoint inhibitors into responsive tumors and the molecule previously underwent Phase 1 clinical testing as a potential adjuvant in healthy volunteers.
Novel antiviral molecules with potential to treat RSV, Influenza and Dengue discovered
Abivax’s screenings of its targeted library of small antiviral molecules have generated positive hits with potential for Respiratory Syncytial Viral (RSV), Influenza and Dengue indications. As part of its long-term collaboration with
On
FINANCIAL CALENDAR
Tuesday April 30, 2019 : Publication and Release of the 2018 Annual Financial ReportFriday June 7, 2019 : Annual Shareholders MeetingThursday September 19, 2019 : Publication of Financial Statements as ofJune 30, 2019 Friday September 27, 2019 : Publication and Release of 2019 Half Year Report
UPCOMING EVENTS:
- BIO Europe SPRING –
March 25 -27, 2019 - Digestive Disease Week (DDW) –
May 18-21, 2019
WEBCAST PRESENTATION
Attendees can participate by weblink (https://edge.media-server.com/m6/p/neya9py8) or connect by phone using the following coordinates:
Telephone conference
Dial in details, Participants:
Confirmation Code: 8987717
| Belgium | 080040905 | ||
| Belgium, Brussels | +32 (0) 1039 1206 | ||
| China | 8008709889 | ||
| France | 0805101655 | ||
| France, Paris | +33 (0) 17 07 32 727 | ||
| Germany | 08000007416 | ||
| Germany, Frankfurt | +49 (0) 6922 224 910 | ||
| Japan | 00531121573 | ||
| Japan, Tokyo | +81 (0) 345 795 720 | ||
| Netherlands | 08000234603 | ||
| Netherlands, Amsterdam | +31 (0) 2071 573 66 | ||
| United Kingdom | 08003767425 | ||
| United Kingdom | +44 (0) 8444 933 857 | ||
| United States | 18668692321 | ||
| United States, New York | +1 917 7200 178 |
About
DISCLAIMER
This press release contains forward-looking statements, forecasts and estimates with respect to certain of the Company's programs. Although the Company believes that its forward-looking statements, forecasts and estimates are based on assumptions and assessments of known and unknown risks, uncertainties and other factors that have been deemed reasonable, such forward-looking statements, forecasts and estimates are subject to a number of risks and uncertainties that could cause actual results to differ materially from those anticipated in such forward-looking statements, forecasts and estimates. A description of these risks, contingencies and uncertainties can be found in the documents filed by the Company with the French Autorité des Marchés Financiers pursuant to its legal obligations including its registration document (Document de Référence). Furthermore, these forward-looking statements, forecasts and estimates are only as of the date of this press release. Readers are cautioned not to place undue reliance on these forward-looking statements.
This press release is for information purposes only, and the information contained herein does not constitute either an offer to sell, or the solicitation of an offer to purchase or subscribe securities of the Company in any jurisdiction, in particular in
View source version on businesswire.com: https://www.businesswire.com/news/home/20190313005922/en/
Source:
Abivax
Finance
Didier Blondel
didier.blondel@abivax.com
+33 1 53 83 08 41
French Media
ALIZE RP
Aurore Gangloff/ Caroline Carmagnol
abivax@alizerp.com
+33 1 44 54 36 66
Investors
LifeSci Advisors
Chris Maggos
chris@lifesciadvisors.com
+41 79 367 6254
US Media
LifeSci Public Relations
Michael Tattory
mtattory@lifescipublicrelations.com
+1 (646) 571-4362
European Media & Investors
MC Services AG
Anne Hennecke
anne.hennecke@mc-services.eu
+49 211 529 252 22
