ABIVAX Reports Impressive 12-month Efficacy and Safety Data from ABX464 Ulcerative Colitis Maintenance Study at United European Gastroenterology Conference
First evidence of long-term efficacy of ABX464 in ulcerative colitis
Endoscopy at Month 12 was performed in 16 / 19 patients, of whom 12 (75%) achieved clinical remission
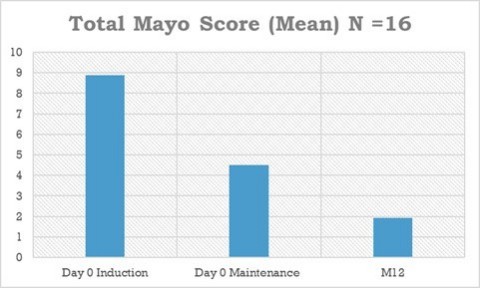
78% reduction of total mayo score, 89% reduction of endoscopic subscore and 97% reduction of fecal calprotectin biomarker (normalized)
Impressive efficacy seen in 8 weeks induction study previously reported is therefore durable or improving in this study
Continued good long-term safety profile
Ulcerative colitis Phase 2b and rheumatoid arthritis Phase 2a clinical studies ongoing, Crohn’s disease Phase 2a planned
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20191020005071/en/
(Graphic: Business Wire)
The oral presentation of the data by Prof. Dr.
Dr Jean-Marc Steens, M.D., Chief Medical Officer of
The one year open-label ABX464 maintenance study was conducted in 22 patients without treatment interruption after completion of the randomised, double-blind, placebo-controlled 8 weeks induction study. A total of 19 patients completed the one year ABX464 open label maintenance study and showed good long-term safety and tolerability of 50mg given orally over 52 weeks.
At month 12, an endoscopy to assess clinical remission status (the critical parameter for regulatory authorities) was performed in 16/19 patients. During treatment with ABX464, patients reduced their total
Detailed analysis showed that of the 7/19 patients in clinical remission at the end of the two-month induction study, 5 patients were still in clinical remission at the end of the maintenance study and 2 patients missed endoscopy and could therefore not be assessed. Of the 12/19 patients NOT in clinical remission at the end of the induction study, 7 patients (58%) achieved clinical remission at the end of the maintenance study, while 4 patients had no remission and 1 had no endoscopy. All 3 patients without endoscopy at 12 months had fecal calprotectin levels in the normal range (<50 microg/g), which is indicative of an absence of intestinal inflammation. All 16 patients with endoscopy showed an endoscopic subscore of 0 or 1, indicative of mucosal healing and in total 12/16 (75%) of the patients undergoing endoscopy achieved clinical remission. These impressive efficacy data make ABX464 a very attractive candidate for further development. Furthermore, the data showed that ABX464 maintained the overexpression of miR124 (a critical factor of immunity and inflammation modulated by ABX464) during the 12-month study period.
Prof. Dr.
Prof. Dr.
ABX464 is a highly differentiated oral drug candidate, with a novel mechanism of action based on the upregulation of a single microRNA (miRNA-124) with anti-inflammatory properties. In addition to the ongoing Phase 2b trial in UC, ABX464 is also being investigated in a Phase 2a trial in rheumatoid arthritis and soon in a phase 2a trial in Crohn’s disease, where its effects could have significant potential.
Details of the oral presentation:
|
Title: |
Oral ABX464 QD is safe and efficacious during 52 weeks open label maintenance following a placebo controlled induction study in ulcerative colitis patients |
|
Presenter: |
Prof. Severine Vermeire, M.D., Ph.D. |
|
Abstract number: |
LB06 |
|
Location and time: |
UEGweek Barcelona Fira Gran Via, Room F3 October 21 at 3:00 p.m. (CEST) |
About ABX464
ABX464 was shown to exert its anti-inflammatory effects through a novel mechanism of action; it binds to the cap binding complex (CBC), which essentially sits at the 5’ end of every RNA molecule in the cell. By binding to the CBC, ABX464 reinforces the biological functions of this complex in cellular RNA biogenesis. Specifically, ABX464 enhances the selective splicing of a single long non-coding RNA to generate the anti-inflammatory microRNA, miR-124, which downregulates pro-inflammatory cytokines and chemokines like TNF-α, IL-6 and MCP-1, thereby “putting a brake” on inflammation and suggesting broad potential as a novel anti-inflammatory therapeutic agent. A seven- to ten-fold increase in miR-124 levels was observed in peripheral blood mononuclear cells (PBMCs) from healthy volunteers upon exposure to ABX464 and also in colorectal biopsies of UC patients treated with ABX464. ABX464 does not impact the splicing of cellular genes.
Webcast Presentation and Teleconference
or connect by phone using the following coordinates:
Telephone conference
Dial in details, Participants:
Confirmation Code: 2536767
|
Belgium |
080040905 |
|
Belgium, Brussels |
+32 (0) 1039 1206 |
|
China, all cities |
400 608 5705 |
|
France |
0805101655 |
|
France, Paris |
+33 (0) 17 07 32 727 |
|
Germany |
08000007416 |
|
Germany, Frankfurt |
+49 (0) 6922 224 910 |
|
Japan |
00531121573 |
|
Japan, Tokyo |
+81 (0) 345 795 720 |
|
Netherlands |
+31 (0) 2071 573 66 |
|
Switzerland |
+41 (0) 445 804 873 |
|
United Kingdom |
08003767425 |
|
United Kingdom |
+44 (0) 8444 933 857 |
|
United States |
+1 866 869 2321 |
|
United States, New York |
+1 917 7200 178 |
About
DISCLAIMER
This press release contains forward-looking statements, forecasts and estimates with respect to certain of the Company's programs. Although the Company believes that its forward-looking statements, forecasts and estimates are based on assumptions and assessments of known and unknown risks, uncertainties and other factors that have been deemed reasonable, such forward-looking statements, forecasts and estimates are subject to a number of risks and uncertainties that could cause actual results to differ materially from those anticipated in such forward-looking statements, forecasts and estimates. A description of these risks, contingencies and uncertainties can be found in the documents filed by the Company with the French Autorité des Marchés Financiers pursuant to its legal obligations including its registration document (Document de Référence). Furthermore, these forward-looking statements, forecasts and estimates are only as of the date of this press release. Readers are cautioned not to place undue reliance on these forward-looking statements.
This press release is for information purposes only, and the information contained herein does not constitute either an offer to sell, or the solicitation of an offer to purchase or subscribe securities of the Company in any jurisdiction, in particular in
View source version on businesswire.com: https://www.businesswire.com/news/home/20191020005071/en/
Source:
Abivax
Communications
Pierre Courteille
pierre.courteille@abivax.com
+33 6 85 34 24 04
Public Relations France
Actifin
Ghislaine Gasparetto
ggasparetto@actifin.fr
+33 1 56 88 11 22
Investors
LifeSci Advisors
Chris Maggos
chris@lifesciadvisors.com
+41 79 367 6254
Public Relations France
Tilder
Marie-Virginie Klein
mv.klein@tilder.com
+33 1 44 14 99 96
Press Relations and Investors Europe
MC Services AG
Anne Hennecke
anne.hennecke@mc-services.eu
+49 211 529 252 22
Public Relations USA
Rooney Partners LLC
Marion Janic
mjanic@rooneyco.com
+1 212 223 4017
